Nano Electrochemistry counts the Blood
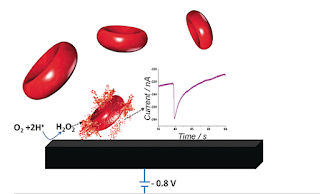
The Scientists at the University of Oxford, UK develop accessible, painless technology as deemed for the development of modern technology. This electrochemical technique replaces the time-consuming Optical Microscope, simplifies the complex instrumentation techniques, and is proved to be an economical technique. The electrochemical instrument required to carry out an electrochemical analysis is an electrode and commercial electrochemical device. Diluted 5 µL of blood placed on biocompatible graphite electrode and voltammograms are obtained. Reduction in the amount of oxygen present in the pure buffer solution results in a slight electrochemical signal at the electrode. Blood samples give a higher intensity signal because of their oxygen content. This electrochemical signal generated gives an estimate of the erythrocyte concentration precisely. This signal strength can further be increased by the addition of Hydrogen peroxide which breaks down into oxygen and water by the e...

