Nano Electrochemistry counts the Blood
The
Scientists at the University of Oxford, UK develop accessible, painless
technology as deemed for the development of modern technology. This
electrochemical technique replaces the time-consuming Optical Microscope,
simplifies the complex instrumentation techniques, and is proved to be an
economical technique.
The
electrochemical instrument required to carry out an electrochemical analysis is
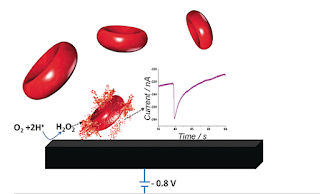
an electrode and commercial electrochemical device. Diluted 5 µL of blood
placed on biocompatible graphite electrode and voltammograms are obtained.
Reduction in the amount of oxygen present in the pure buffer solution results
in a slight electrochemical signal at the electrode. Blood samples give a
higher intensity signal because of their oxygen content. This electrochemical
signal generated gives an estimate of the erythrocyte concentration precisely.
This
signal strength can further be increased by the addition of Hydrogen peroxide
which breaks down into oxygen and water by the enzymes glutathione peroxidase.
This time the carbon microelectrode was replaced by graphite electrode the
current was recorded keeping the voltage as a constant. This measurement
resulted in spikes with an increase in the erythrocyte concentration.
Have a Nano
Electrochemical research work to be published?? The arena is all yours at
Materials Electrochemistry 2018.
Reference:
Electrochemical Red Blood Cell Counting: One at a Time

Comments
Post a Comment